What Happens When the Last AMR Researcher Turns Off the Lights in the Lab?
Much of modern medicine is possible because of antibiotics. Orthopedic surgeries, caesarean deliveries, and heart and kidney transplants are made safer by antibiotics. Patients who are immunocompromised rely on antibiotics to avoid devastating infections, and those with uncomplicated cystitis, pneumonia and cellulitis feel better after a single course of these amazing products.
Just about every person, from newborns to the great-grandparents who can’t wait to hold them, will need an antibiotic at some time in their lives.
But every ID specialist recognizes antibiotics have a limited shelf life because exposure to antibiotics selects for resistant organisms. Infections caused by antimicrobial resistant organisms is one of the most serious medical problems facing the world today, causing more than 1.27 million deaths per year—more than 35,000 of them in the United States, according to the WHO and CDC. Yet, much of the world will not realize there is a problem until the last AMR researcher turns off the lights in the lab.
This precious resource needs to be constantly renewed. “The antimicrobial ecosystem is fundamentally unique,” said Emily Wheeler, the senior director of infectious disease policy at the Biotechnology Innovation Organization (BIO), and the director of the “Working to Fight AMR” public awareness campaign. “The bacterial and fungal pathogens that are behind the most threatening resistant infections are evolving and adapting constantly to resist the drugs that are available to treat them, and that is why we need to continue innovation in this space to keep up with resistance as it grows.”
However, only two of the top 50 pharmaceutical companies have an antibiotic in clinical development, according to the Pew Charitable Trusts (bit.ly/46AuTlr-IDSE). Therefore, research and development (R&D) falls primarily to small companies, which struggle to bring an antibiotic to market, only to fail after the launch—seven of 12 companies that recently developed an antibiotic went under after the FDA approved the medication, according to a 2022 BIO report, resulting in an exodus from the ID R&D laboratories (bit.ly/3yGqBwl-IDSE).
That exodus is real, according to a new report by the AMR Industry Alliance. The report estimated that there are only about 3,000 people in antimicrobial development research globally (bit.ly/3SHvz2V-IDSE). To find this estimate, the authors looked at a number of sources, including the AMR R&D Hub, PubMed and patent data, to develop a range of researchers (1,218 to 4,746) they consider to be people who were conducting research in AMR and developing new antimicrobials, and then averaged the two numbers.
“It’s impossible to directly count every researcher in the space, but we thought this was a fair way of estimating the size of the workforce using the data that are currently available,” explained Daniel O’Keefe, a spokesperson for the AMR Industry Alliance.
“The problem is the lack of reimbursement once these drugs come onto the market,” explained Kevin Outterson, JD, LLM, the Austin B. Flecher Professor of Law at Boston University School of Law. “The companies are going bankrupt because hospitals are not using [the new antibiotics] or they are trying to pay generic pricing [for them].”
Antibiotics are ubiquitous in society, and people have expectations about them that do not match the reality of the marketplace, added Jennifer Leeds, PhD, who recently retired. Dr. Leeds was the head of antibacterial discovery for Infectious Diseases at the Novartis Institutes for Biomedical Research before Novartis pulled out of ID, when she had to dismantle the lab and move to another division of the company. During her tenure, she co-invented and co-led an international discovery team for the novel antibacterial LFF571, a semisynthetic thiopeptide with activity against Clostridioides difficile, but was never brought to market by the company.
“You are fighting not just resistance, but also perception,” Dr. Leeds said. “People expect antibiotics to be cheap and readily available and super safe because it is one of the only classes of medications that everybody from birth through death needs and receives.”
Hospitals balk at paying for expensive branded antibiotics that could cost upward of $20,000 per course, but they willingly pay $200,000 or more for a cancer drug, and antibiotics do not come close to the cost of some orphan drugs with price tags of $1 million or $2 million (bit.ly/4dhWavH-IDSE).
“There is a disconnect regarding the value that antibiotics truly provide to human health,” Ms. Wheeler said. “I don’t think that the recognition is there that all of modern medicine lies on the backs of antibiotics, and antibiotics are critical to ensuring many medical procedures can happen.”
Since older generic antibiotics have generally been inexpensive and effective until resistant organisms affected their utility, there is now an unwillingness to pay the price of the newer more expensive antibiotics developed to combat that resistance.
This disconnect between the value of antibiotics and what people expect to pay for them is one reason AMR researchers are leaving the lab, according to Mr. Outterson, who is also the executive director of CARB-X, which provides push funding to support the development of new antimicrobials. “The market reimbursement piece is broken, and therefore the companies that are doing the research are constrained. That’s why people leave the field.”
People in every aspect of ID are passionate about their field, Dr. Leeds said, but it is not enough. New graduates must consider their financial health.
“The challenge is money,” she said. “Money drives everything. It drives investments. It drives compensation for talent. It drives retention. It drives interest for people to get into a field. Scientists are in a competitive space, and so attracting not just physicians, but scientists, into the infectious disease area when there are other choices they can make, is difficult.”
Even if they find a position with a laboratory that is researching antimicrobials, it does not mean they will grow in that position. “Eighty-two percent of the people who had worked in antibacterial R&D in June 2018, five years later were not [working in the field],” Mr. Outterson said (see figure).
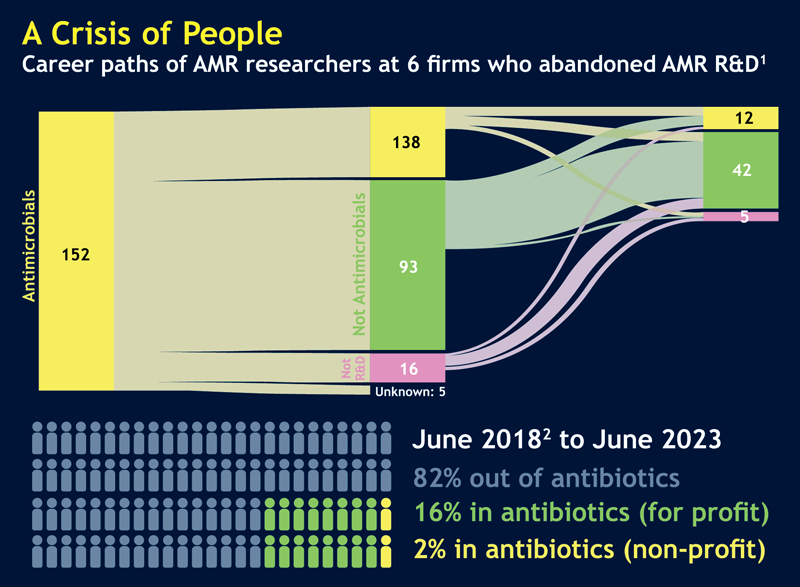
A Brain Drain
Attracting new graduates isn’t the only challenge for R&D. The departure of experienced, talented people means that young scientists are losing mentors who have accumulated a lifetime of experience and knowledge.
“Infectious disease R&D and clinical practice is pretty much the only area of science and medicine that gets harder the longer you stay in it because you are always facing the challenge of erosion of drugs due to resistance,” Dr. Leeds said.
She described the typical scenario. “Usually [a job] gets easier. You learn more. You perfect paradigms. You have better diagnostics and so on. You can double or triple drugs if you need to, and you can find ways to deal with safety concerns. [But] in the ID space, you had good coverage; now it’s going away.”
All too often, people reinvent the wheel because they don’t have an experienced investigator to explain “that work was done 25 years ago in a previous lab, and the reasons why it did not work,” she added.
“It’s not to say that people should not try to improve on things, but there are certain fundamental things that are going to just fail, and they fail for real scientific reasons,” Dr. Leeds said. “But there is not enough mentoring, and there is not enough exposure to people with a lot of experience because most of us have left the field or retired, or we’re burned out.”
The science behind ID research has excellent predictive translational models, a good drug discovery and development perspective, and extensive and diverse organisms to test against. “If your phase 1 looks good, the chances of having a successful development path are quite high—one of the highest in medicine,” Dr. Leeds said. But young researchers are missing the lead investigators who know where efforts should be made.
There is a deep well of knowledge that is being lost, according to Dr. Leeds. “Both on the clinical side and the R&D side, the talent drain has been substantial.”
And it takes time to build a good team with not just experienced lead investigators, but talented post-docs and skilled lab technicians and research assistants. Who wants to join a promising team if they won’t have a job in five years, and what lead investigator wants to dismantle this team when the money runs out? Having experienced it at Novartis, Dr. Leeds said that is emotionally harder than putting the team together.
“Many experts with essential knowledge have already exited the field, and those who remain face cost pressures, limited incentives and a lack of compelling job, career and research opportunities,” the Alliance report stated. This brain drain may complicate every aspect of antimicrobial research, from basic science to the expertise needed within agencies like the FDA to ensure that antimicrobials are safe and effective.
Multifaceted Approach to Solutions
Just as a clinician might use a combination of antimicrobials to overcome the various mechanisms that bacteria use to resist them, the problem of supporting the development and marketing of anti- microbials and the people who research them will take a multipronged approach, the experts said.
Push and pull incentives are needed, the experts noted. Push incentives have been successful in helping organizations like CARB-X provide incentives to small companies to support the R&D pipeline. Other programs like ICARe (Interdisciplinary Course on Antibiotics and Resistance) and Future Leaders Against AMR are helping researchers early in their careers to gain experience in antimicrobial research. These and other initiatives that support research and the people who work in this field need to continue, according to the Alliance report.
Pull incentives include new payment models once antimicrobials are developed and approved, Mr. Outterson explained. The PASTEUR (Pioneering Antimicrobial Subscriptions to End Upsurging Resistance) Act, which has bipartisan support, would institute a subscription model like the ones used by streaming services for videos, podcasts or music. Many people believe this is one way to make sure antibiotics are valued appropriately.
PASTEUR is one example of a “delinkage model” that supports R&D for a successful product without requiring sales price and volume to recoup that money before seeing a profit (Clin Infect Dis 2021;73[11]:e4451-e4453).
And the paradigm about antibiotic costs needs to shift, added Dr. Leeds. Doesn’t an infection caused by an organism that is resistant to every antibiotic available deserve some type of orphan status? she asked.
There are signs that people are recognizing the need to keep the laboratories running to maintain the pipeline for antimicrobials. The UN General Assembly will convene a second high-level meeting on AMR in New York in September. The first high-level meeting on AMR was in 2016, and it resulted in efforts like CARB-X. A multiple-stakeholder meeting was held in May, and experts like Mr. Outterson hope this will lead to more solutions. The UN is holding this meeting because a secure antimicrobial pipeline is a global security issue affecting health, food security and sustainable development, the organization said.
