In Action

research & science
Invest in R&D to meet public health needs with new innovative diagnostics and treatments
Read a summary of our work in 2022 here

appropriate use
Work to reduce the development of antimicrobial resistance
Read a summary of our work in 2022 here

access
Improve access to high-quality antibiotics and ensuring that new ones are available to all
Read a summary of our work in 2022 here

manufacturing
Reduce the environmental impact of manufacturing
Read a summary of our work in 2022 here


ANTIBIOTIC ADJUVANT’S ELECTRONIC TOOL REDUCES ANTIBIOTIC MISUSE IN THE LONG-TERM CARE SECTOR
...


BOEHRINGER INGELHEIM WORKS WITH SUPPLIERS TO END USE OF BIOMASS WASTE AS FERTILIZER
...


Mylan – Implementing ‘Zero Liquid Discharge’ at Manufacturing Facilities in India
One main cause of the rise of AMR is the over-prescribing and inappropriate use of antibiotics in or by humans, as well as improper disposal. Another main cause is the use of antibiotics in intensive...
Novartis – KAF156 Molecule
KAF156 was discovered and is being developed to address the lack of new molecules active against malaria parasites. Next-generation antimalarials are urgently needed to secure the gains made over the...


AEQUOR’S NOVEL MOLECULES COUNTER BIOFILM ASSOCIATED WITH HOSPITAL-ACQUIRED INFECTIONS
Bacteria and fungi can form biofilms that stick to surfaces inside the human body. This process enables microbial cells to become tolerant and resistant to antibiotics, thus making infections more dif...


AiCuris Initiates Clinical Development of AIC499, a Novel Resistance-Breaking Antibiotic against a Broad Range of MDR Gram-Negative Bacteria
With the rapid emergence and spread of MDR bacterial infections, antimicrobial resistance (AMR) has become a major global public health threat. Patients in intensive care units (ICUs) are particularly...


ANTABIO – PEi Program
Antimicrobial resistance affects a high percentage of patients suffering from cystic fibrosis. This happens due to a combination of a variety of factors, which includes physiological changes. Thus, in...


BD – “Resistance Fighter” Awareness and Mobilization Campaign
This campaign addresses the need to raise global awareness about antimicrobial resistance (AMR) and the important roles that everyone plays in the fight against its spread. This campaign targets all w...


BD – AMR Education and Training Collaboration with the London School of Hygiene and Tropical Medicine (LSHTM)
Need to build training programs and capacity building for continuous education to help raise awareness of AMR and improve diagnostic utilization....

BD – Phoenix™ M50 System
Targeted antimicrobial therapy. Wrong diagnosis can lead to wrong therapies, which in turn delay treatment and can cause AMR...


BIOMÉRIEUX LEADS EUROPEAN EFFORT TO QUANTIFY DIAGNOSTICS’ VALUE TO COMBAT AMR
...


CENTRIENT AND CIPLA COMBAT IRRATIONAL ANTIBIOTIC USE IN CHINA AND INDIA
Inappropriate and overuse of antibiotics in the world’s two most populous countries pose a danger to their populations. In China, where consumption of antimicrobials is trending downward, irratio...


DSM – Sustainably-produced Antibiotics
Common practice in today’s pharmaceutical manufacturing industry requires large volumes of chemical solvents, produces millions of tonnes of CO2 emissions, and could contribute to the growing threat...


GSK – Improving Access to Antibiotics and Vaccines
GSK recognizes that access to medicines and healthcare should be available to everyone who needs them, no matter where they live or how much they can afford. In many countries, we still need to improv...


GSK – Responsible Antibiotic Manufacturing
GSK recognises that factory discharges of antibiotics and antimicrobial active materials could contribute to an increase in resistant genes in the environment. GSK is committed to ensuring that discha...

GSK – Survey of Antibiotic Resistance (SOAR)
Understanding local antibiotic drug resistance patterns helps encourage appropriate antibiotic usage; supports development of local antibiotic prescribing guidelines and vaccination strategies; and, m...


GSK DRIVES MANUFACTURING ENVIRONMENTAL STANDARDS
...

Johnson & Johnson – Access to diagnostics and therapeutic solutions for TB and MDR-TB (with FIND Diagnostics)
While MDR-TB is threatening the global effort to eliminate TB, the high rates of missed or delayed diagnosis are preventing prompt, effective treatment and allowing MDR-TB’s continued spread. The ne...


SIRTURO® (bedaquiline) Development and Launch
Globally, more people die of tuberculosis than any other infectious disease. Drug resistant tuberculosis (MDR-TB) accounts for roughly one-third of all AMR fatalities. Each year, roughly 500,000 peopl...


Merck & Co., Inc – Partnering with Local Hospitals Worldwide to Implement Antimicrobial Stewardship (AMS) Programs
Any use of antimicrobials contributes to the development of resistance, but widespread unnecessary and excessive use makes it worse. AMS refers to a collection of interventions geared toward optimizin...


MSD AND THE WELLCOME TRUST INVEST IN TRANSFORMATIVE AFFORDABLE VACCINES
...



MOBIDIAG’S FAST SCREENING TEST IDENTIFIES RESISTANT BACTERIA, ASSESSES PATIENT CONDITION
...


Otsuka – FighTBack Initiative
Considering the dramatic rise in bacterial resistance worldwide, rational use of the MDR-TB compound delamanid is critical to ensuring that the greatest number of patients have access to this medicine...


Otsuka – MDR-Tuberculosis R&D
TB bacteria are so resilient that patients must take a regimen of several different medicines for anywhere from six months to a year. In the case of drug-resistant strains such as multidrug-resistant...


Peptilogics Receives Qualified Infectious Disease Product Designation from FDA for Lead Compound PLG0206 as a Treatment for Prosthetic Joint Infection
Prosthetic Joint Infection (PJI) is one of the most challenging complications of joint arthroplasty and a serious, life threatening condition with few effective treatment options. “Total joint re...

Pfizer – Antimicrobial Access Programs
At Pfizer we believe that access to quality healthcare and the opportunity to lead healthy lives is an extremely important social goal. The power and value of collaboration between public and private...


Pfizer – Antimicrobial Innovative Vaccines
Pfizer has made significant and substantial investments in vaccine R&D to develop new vaccines and to support several vaccines already in clinical usage. It has been clearly demonstrated that bact...


Pfizer – Massive Open Online Course on Stewardship
Pfizer routinely sponsors and conducts medical education outreach to healthcare providers through a variety of mechanisms, including medical symposia and Pfizer sponsored preceptorships, to ensure app...


PFIZER HELPS DELIVER MEDICAL PRODUCTS BY DRONE IN GHANA
...

Pfizer, ICMR to Establish a Centre in New Delhi to Combat AMR
The project will help address the growing threat of antimicrobial resistance (AMR) in India, as it is important to invest all the necessary resources in combating the adverse impact of AMR, due to the...


SANDOZ DRIVES REMEDIATION PROGRAM TO HELP MAINTAIN GLOBAL SUPPLIES OF CRITICAL ANTIBIOTIC
...
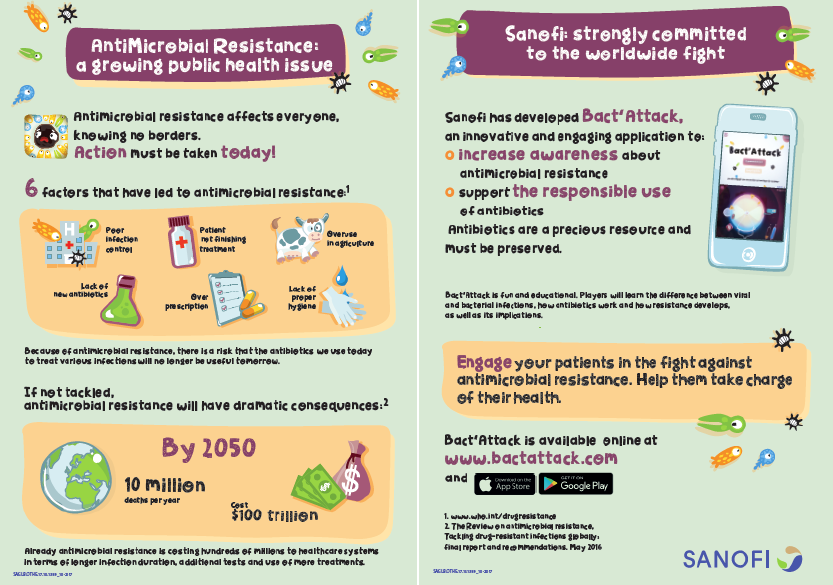

BACT’ATTACK®
By 2050, if considerable gains are not made to combat AMR, it could become the main cause of death among all diseases worldwide. In addition to promoting and fostering R&D for AMR-related health p...


Shionogi – Antimicrobial Stewardship Program
At Shionogi, antimicrobial stewardship means selecting the best medicine and administration method and ensuring treatment starts and ends at the optimal time, in order to make treatment safe and relia...