AMR Industry Declaration

Taking collective action
We support the increasing recognition that the value assigned to antibiotics and diagnostics often does not reflect the benefits they bring to society, nor the investment required for their creation. Therefore, we call on governments to commit to allocating the funds needed to create a sustainable and predictable market for these technologies while also implementing the measures needed to safeguard the effectiveness of antibiotics. Specifically: Creating a sustainable and predictable market. We call for governments to commit funding and support the development and implementation of transformational commercial models that (a) enhance conservation of new and existing antibiotics, while (b) improving financial and access-related predictability for both industry and health systems.

Enhancing conservation
We support measures for the prevention of infection along with conservation and appropriate use of all antibiotics, including:
- Implementation of the WHO’s Global Action Plan calling for comprehensive stewardship programmes and activities that enhance health system capability to use antibiotics appropriately.
- Enhanced integration of fast and accurate point-of-care and laboratory diagnostics with antibiotics to ensure appropriate use of antibiotics for the patients who need them. To enable this, we call for improved reimbursement and use of advanced diagnostics.
- Furthermore, we call for governments, insurers, healthcare providers and other health system stewards to remove financial incentives for individuals (such as doctors, veterinarians and pharmacists) or institutions that reward the prescribing of antibiotics in greater volumes.

Improving financial and access-related predictability
For both Industry and health systems is required to ensure sustainable investment in new antibiotics and diagnostics. To this end, we welcome appropriate incentives, coupled with safeguards to sustain the effectiveness of new and existing antibiotics. We believe two fundamental approaches are needed to accomplish these goals:
- We welcome proposals that (a) support reduction in the link between financial revenues for new antibiotics and the amount they get used while (b) mitigating the financial risk for both developers and health systems. As different jurisdictions may require different solutions, a range of approaches to creating such delinkage will likely need to be utilised. Possible approaches include the system of lump sum Market Entry Rewards proposed by the Review on AMR, insurance-like purchase models, and novel intellectual property-based approaches with appropriate safeguards. An integral part of these models is a reduced need for promotional activity from companies.
- We also support the principle that in developed markets, prompt reimbursement decisions at prices that reflect value should be provided for new drugs and diagnostics to reflect the benefits they bring (with measures for stewardship to prevent misuse) – as also acknowledged in the work of the Review on AMR. This calls for funding to be allocated and for payers to appropriately assess and value innovative antibiotics and diagnostics, in line with the good progress that has been made by regulatory authorities

Global coordination, local action
We call for a global commitment to coordinated action on stewardship, conservation, hygiene, and the creation and use of new commercial models for antibiotics and diagnostics. As noted above, we recognise different models may be appropriate for different countries, health systems, and products. All parties should commit to allocating funding and finding paths that work for their situation. We are ready to work stepwise with countries to implement such models.
Commitments by signatory companies
The under-signed companies are already actively engaged in combating AMR as appropriate to their business. We stand ready to work in partnership with leading countries to deliver sustainable solutions to meet this global challenge. We invite other companies to join this Declaration and comments from all other stakeholders are welcome. We will review and update the Declaration every 2 years, to reflect progress and changing priorities. We commit to:
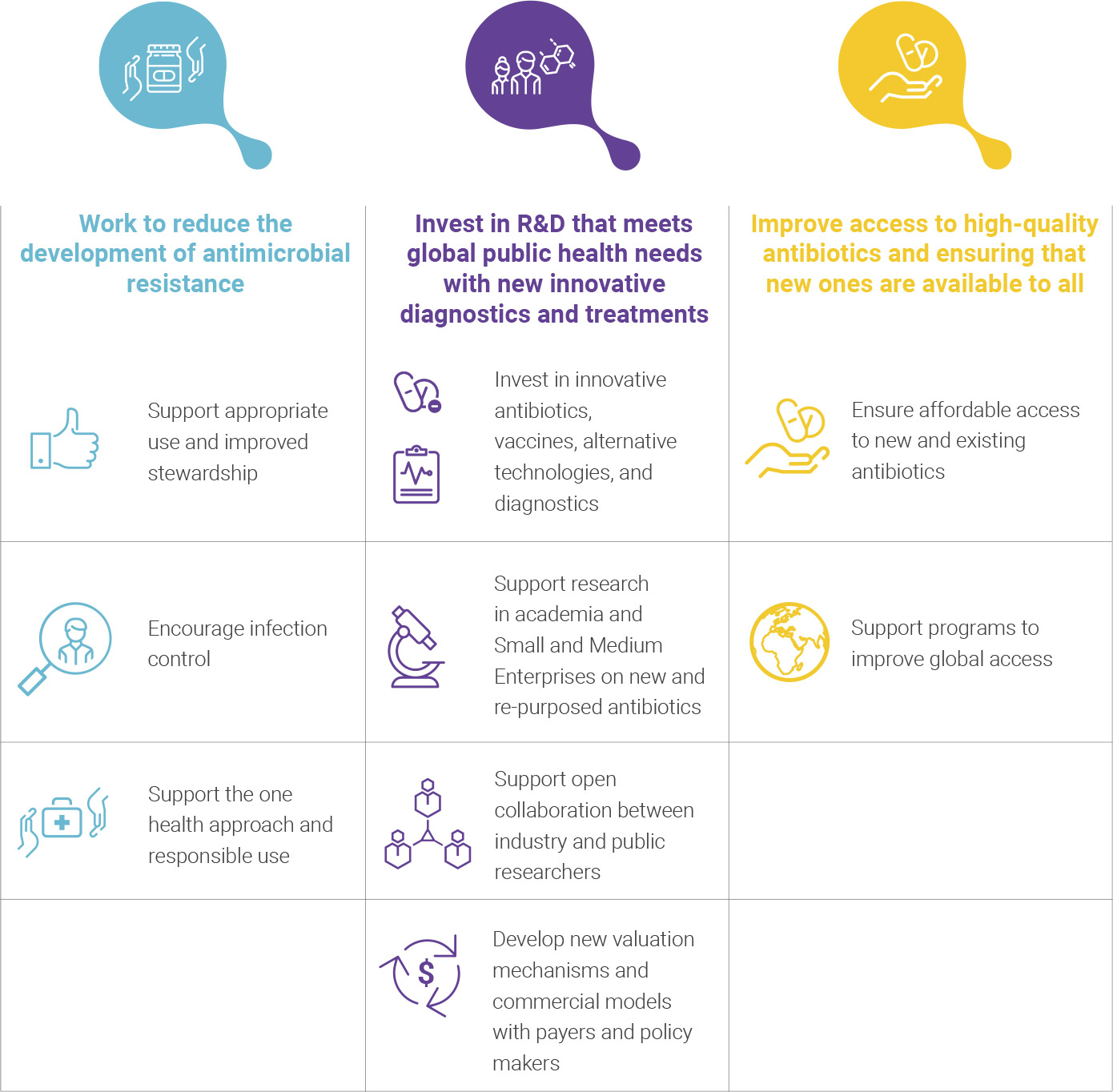

Work to reduce the development of antimicrobial resistance
- We are committed to antibiotics only being used in patients who need them, we support continued education for clinical professionals on appropriate use, and we welcome the WHO Global Action Plan’s focus on improved stewardship.
- We encourage infection control via improved hygiene, vaccination, and preventive treatments to help reduce the number of infections needing antibiotic treatment.
- We support measures to reduce environmental pollution from antibiotics, along with a ‘one health’ approach towards prudent and responsible use, including a global reduction of unnecessary antibiotic use in livestock, and we applaud moves from major food groups to work towards this goal.

Invest in R&D to meet public health needs with new innovative diagnostics & treatments
- We are investing in a range of innovative antibiotics, vaccines, alternative technologies, and diagnostics for resistant infections. We are advancing our pipelines, but more work and investment into multiple approaches is needed to overcome the significant scientific difficulties of antibiotic discovery.
- We will continue to support research in academia and SMEs on new and re-purposed antibiotics. We welcome proposals to increase investment via coordinated global routes in efforts to develop useful diagnostics, antibiotics, vaccines, and alternative technologies.
- We support new ways of working such as open collaborations between industry and public researchers to overcome the scientific challenges of creating new antibiotics and diagnostics. Collaborative public-private projects already demonstrate what we can achieve together, but more can be done: several companies co-established the New Drugs for Bad Bugs (ND4BB) programme as part of IMI with the European Commission and others are actively engaging in collaborations funded in the US by BARDA and the NIH.
- As acknowledged, the value assigned to antibiotics and diagnostics often does not reflect the investment required for their creation or the benefits they bring to society, and we stand ready to work with payers and policymakers on new valuation mechanisms and commercial models that specifically address the unique challenges of this market.

Improve access to high-quality antibiotics and ensuring that new ones are available to all
- As part of the WHO Global Action Plan’s proposal for a comprehensive program of sanitation, hygiene, vaccination, infection control, education, and stewardship, we support mechanisms to ensure affordable access to new and existing antibiotics to the patients who need them, in all parts of the world and at all levels of income.
- We recognise the success of programmes to improve global access to drugs in HIV, TB, and malaria and call for a similar collaborative effort to address issues of access to antibiotics.
